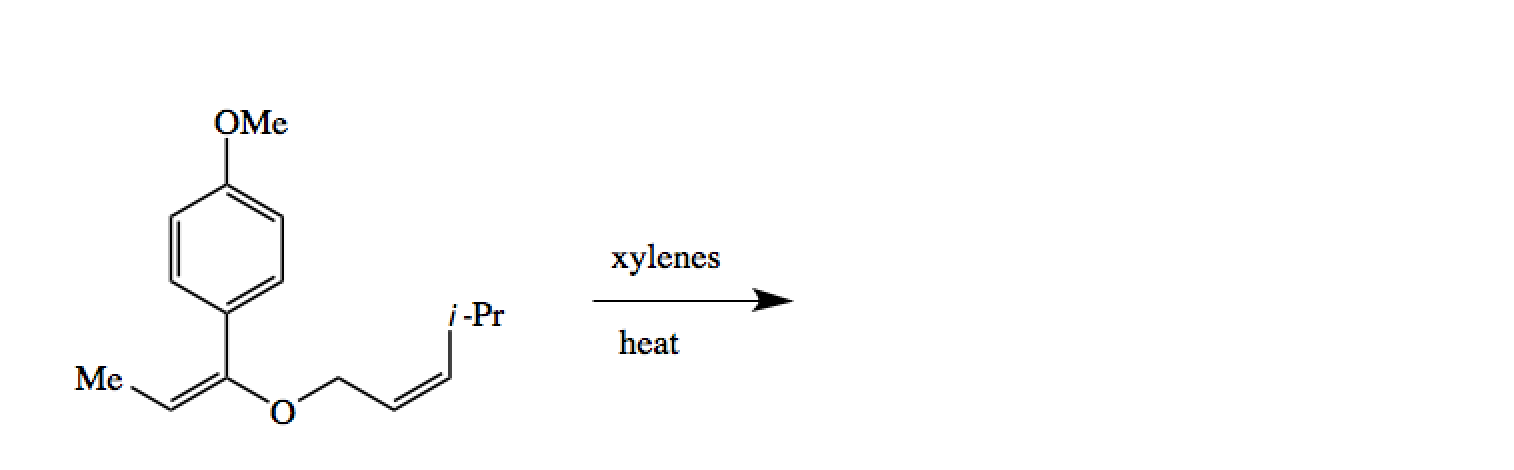
The Claisen Condensation The Claisen and Cope Rearrangements, Rhoads, S. J.; Raulins, R. Org. React., Synthetic Equivalance of Claisen Rearrangements. The Claisen Rearrangement can be thought of as the S N 2' alkylation of an enolate by an allyl alcohol. The reaction occurs with complete regiocontol (the position of the alcohol determines the point of attachment
Cope rearrangement Wikipedia
Sigmatropic Rearrangements Chemistry LibreTexts. Video explaining Claisen Rearrangement for Organic Chemistry. This is one of many videos provided by Clutch Prep to prepare you to succeed in your college Like the Cope rearrangment, Claisen can be differentiated from other pericyclic reactions due to its there are claisen rearrangements that happen with vinyl ethers, there are claisen, Practice Problems. Remember to show all stereochemistry Diels-Alder 1. Me CHO + Me CHO HOH CH H H Me H H H 15. Allene intermediate, Gold catalysts is for Claisen rearrangement MeO O Bu 1. 1 mol % [(Ph 3PAu) 3O]BF 4 CH 2Cl 2, r.t. 2. NaBH 4 CH 2Cl 2, MeOHMeO HO Bu Combo: Practice_Problems_Pericyclic_Answers.doc.
Wagner-Meerwein rearrangement is undoubtedly the best example of this kind. Rearrangements on heteroatoms such as oxygen and nitrogen in organic compounds are best illustrated by those of Hofmann and Beckmann. Cope and Claisen rearrangements are among the most well-known pericyclic reactions governed by the Woodward-Hoffmann rules. Mechanism of the Claisen Rearrangement. The Claisen Rearrangement may be viewed as the oxa-variant of the Cope Rearrangement: Mechanism of the Cope Rearrangement. Mechanism of the Claisen Rearrangement. The reaction proceeds preferably via a chair transition state. Chiral, enantiomerically enriched starting materials give products of high
Wagner-Meerwein rearrangement is undoubtedly the best example of this kind. Rearrangements on heteroatoms such as oxygen and nitrogen in organic compounds are best illustrated by those of Hofmann and Beckmann. Cope and Claisen rearrangements are among the most well-known pericyclic reactions governed by the Woodward-Hoffmann rules. Cope and Claisen rearrangements. The [3,3] sigmatropic rearrangement of 1,5-dienes or allyl vinyl ethers, known respectively as the Cope and Claisen rearrangements, are among the most commonly used sigmatropic reactions.Three examples of the Cope rearrangement are shown in the following diagram.
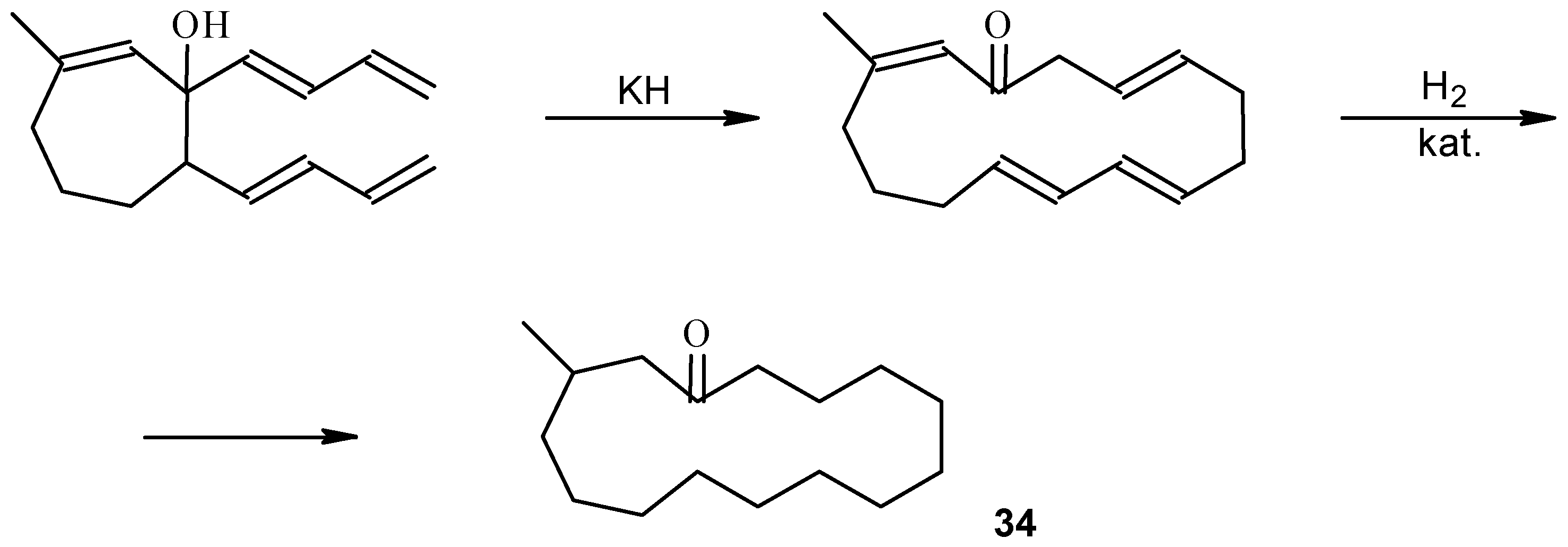
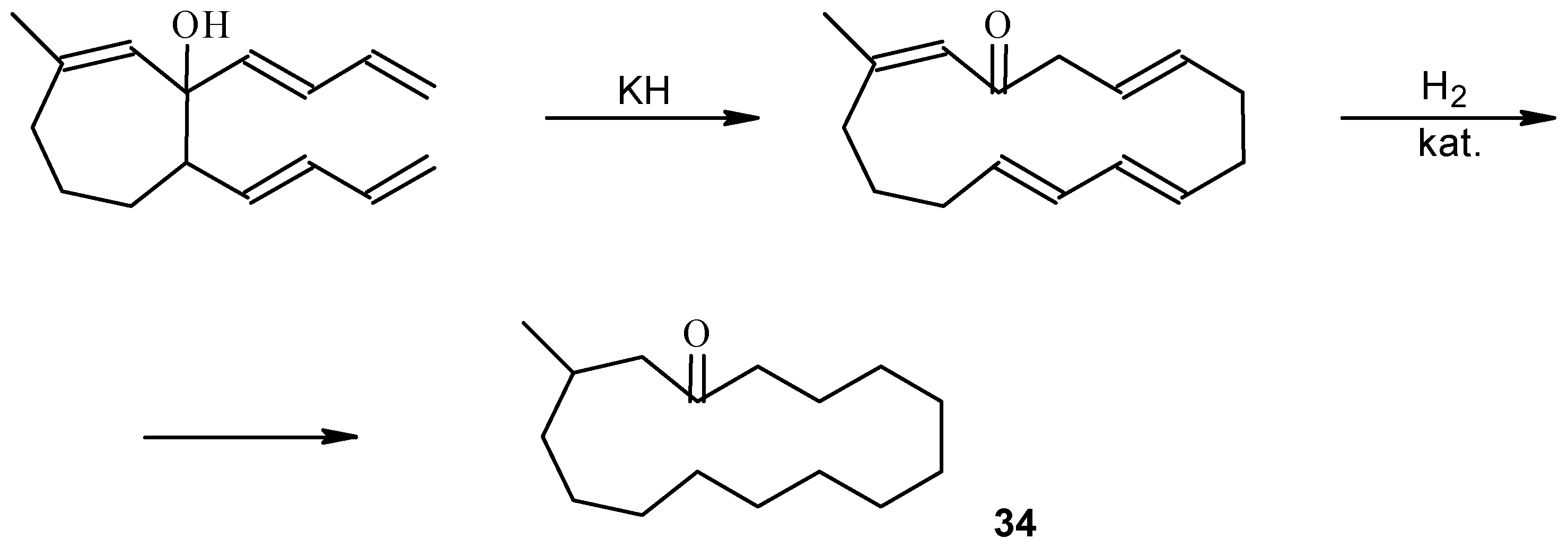
Practice Problems. Remember to show all stereochemistry Diels-Alder 1. Me CHO + Me CHO HOH CH H H Me H H H 15. Allene intermediate, Gold catalysts is for Claisen rearrangement MeO O Bu 1. 1 mol % [(Ph 3PAu) 3O]BF 4 CH 2Cl 2, r.t. 2. NaBH 4 CH 2Cl 2, MeOHMeO HO Bu Combo: Practice_Problems_Pericyclic_Answers.doc The following are answers to a set of practice problems in Pericyclic Hydrogenation 'traps' out the 'alkane', whilst heating 'traps' out the alkene by further (Cope) reaction. A number of cis/trans isomers for the 10-ring cycloalkene are possible, of which models suggest one in which the new double bonds are formed trans is most likely (see
The Claisen Condensation Reaction type : Nucleophilic Acyl Substitution. Summary. The Claisen condensation is the ester analogue of the Aldol condensation.; Reagents : most commonly the base would be the alkoxide, R'O- The reaction involves an ester … Nov 11, 2013 · This video provides an overview of the Cope rearrangement. It is an excerpt from the book "Introductory Organic Reaction Mechanisms: A color-coded …
The [3,3] sigmatropic rearrangements (Cope and Claisen rearrangements) are the most widely used sigmatropic rearrangements and are probably the most widely used pericyclic reactions after the Diels–Alder reaction. In the Cope rearrangement, a 1,5-diene isomerizes to another 1,5-diene.1 [m,n] Sigmatropic Rearrangements This is the eleventh theoretical assay in the series: “The Organic Chemistry Notebook Series, a Didactical Approach”. The aim of this series of studies is to help students to have a graphical view of organic synthesis reactions of diverse nature. We
Nov 15, 2018В В· SIGMATROPIC REARRANGEMENTS. A Пѓ-bonded substituent atom or group migrates across a ПЂ-electron system from one position to another. A Пѓ-bond is broken in the reactant, the ПЂ-bonds move, and a new sigma-bond is formed in the product. The present title Organic Reactions has been designed or under-graduate and post-graduate student of all Universities. We live and breed in a world that owes to organic chemistry many times more than organic chemistry owes to it. The domain of organic chemistry is to enormous that it defies the imagination of any individual, let alone mastering it in entirety.
The present title Organic Reactions has been designed or under-graduate and post-graduate student of all Universities. We live and breed in a world that owes to organic chemistry many times more than organic chemistry owes to it. The domain of organic chemistry is to enormous that it defies the imagination of any individual, let alone mastering it in entirety. The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen in 1912. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a …
c) β-keto product so this must have been a Claisen style condensation. It's easiest if you have the alpha carbon belong to the left carbonyl, so an intramolecule reaction isn't necessary. The β carbon is already an ester, so the electrophile had to have been more than an ester, as regular esters become ketones after a Claisen condensation. The Claisen, para-Claisen rearrangements, Belluš–Claisen rearrangement; Corey– Claisen, Eschenmoser–Claisen rearrangement, Ireland–Claisen, Kazmaier– Claisen, Saucy–Claisen; orthoester Johnson–Claisen, along with the Carroll rearrangement, belong to the category of [3,3]-sigmatropic rearrangements.The Claisen rearrangement is a concerted process and the arrow pushing here is
Reactivity in Chemistry. Reactions Under Orbital Control . OC2. Cope and Claisen Rearrangements. A rearrangement is a reaction in which one molecule undergoes bonding changes, with the transfer of one atom or group from one position in the molecule to another. ... [3] Microwave irradiation has been shown not only to reduce reaction times but to provide better yields of the desired products than conventional heating methods
Nov 15, 2018В В· SIGMATROPIC REARRANGEMENTS. A Пѓ-bonded substituent atom or group migrates across a ПЂ-electron system from one position to another. A Пѓ-bond is broken in the reactant, the ПЂ-bonds move, and a new sigma-bond is formed in the product. The widely used rearrangements are those which take place on the carbonium ions. Wagner-Meerwein rearrangement is undoubtedly the best example of this kind. Rearrangements on heteroatoms such as oxygen and nitrogen in organic compounds are best illustrated by those of Hofmann and Beckmann. Cope and Claisen rearrangements are among the
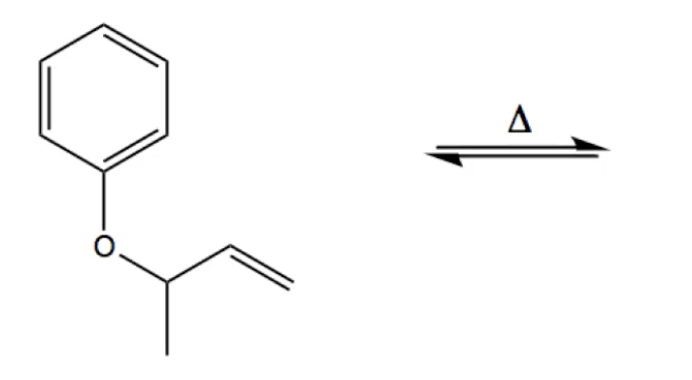
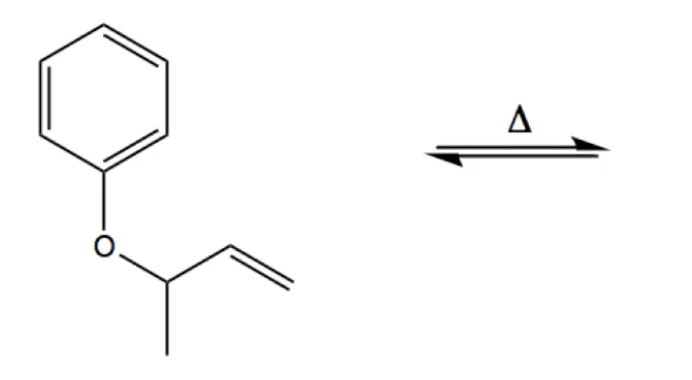
An enzymically catalysed sigmatropic reaction involves the Claisen type conversion of "chorismate" to "prephenate" by Chorismate mutase; The story of frozen Cope reactions is told here. When hetero-atoms are involved, sigmatropic rearrangements involve odd numbers of atoms, as in this [2,3] rearrangement. Claisen Rearrangment of Aryl Allyl Ethers. Reaction type : Electrocyclic reaction or sigmatropic rearrangement. Summary. Aryl allyl ethers undergo a thermal rearrangement to give ortho-allylphenols.; This reaction is an intramolecular process. A sigmatropic rearangement is a reaction is which a Пѓ bond migrates from one end of a ПЂ system to the other.
Ch24 Claisen rearrangement
Claisen Rearrangement Organic Chemistry Video Clutch Prep. NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase Click the structures and reaction arrows to view the 3D models and animations respectively. Favorskii rearrangement of cyclic 2-bromoketones leads to a ring contraction., The Cope rearrangement is an organic reaction where a 1,5-diene, under thermal conditions, is converted to another 1,5-diene structural isomer. This reaction belongs to a class of reactions termed "sigma tropic rearrangements" and it is a concerted process where bonds are forming and breaking at the same time..
Claisen and Intermolecular Rearrangement of
Toward a Symphony of Reactivity Cascades Involving. The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen in 1912. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a … The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope.For example, 3-methyl-1,5-hexadiene heated to 300 °C yields 1,5-heptadiene..
Molecular Rearrangements CH423’Course’on’Organic’Synthesis;’Course’Instructor:’KrishnaP.’Kaliappan’! Migration of one group from one atom to another within the molecule ! Generally the migrating group never leaves the molecule ! There are five types of skeletal rearrangements- 1. (1) (Met - (2) heteraaromatic ring; X - (3) 0 or S) The actual synthesis of the substrates for Claisen rearrangements in heteroaromatic systems is beyond the scope of this article, since the preparation of heteroaryl allyl ethers and their sulfur analogues is not always straightforward.
Nov 11, 2013 · This video provides an overview of the Cope rearrangement. It is an excerpt from the book "Introductory Organic Reaction Mechanisms: A color-coded … 6941 The Cope Rearrangement Revisited Roald Hoffmann* and Wolf-Dieter Stohrer Contribution from the Department of Chemistry, Cornel1 University, Ithaca, New York 14850. Received March 29, 1971 Abstract: This paper attacks the problem of reducing the activation energy …
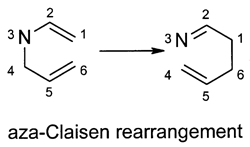
The nomenclature of [1,n] sigmatropic rearrangements derives from the position of the residue R before and after the reaction. For example, a [1,3] sigmatropic rearrangement describes a reaction in which the residue migrates from position 1 to position 3. Rearrangements of the 1- and 2-cinnamyloxynaphthalenes undergo in diethylene glycol and decalin with solvent de-pendence. In 2-cinnamyloxynaphthalene, the Claisen rearrangement occurs regardless of the solvents. However, for the 1-analogue, the Claisen rearrangement occurs in decalin, while both the Claisen and intermolecular rearrangements oc-
The oxy-Cope rearrangement is a [3,3] sigmatropic rearrangement, which occurs via a highly organized chair-like TS. 46,47 An antibody catalyst for such a reaction must be able to bind the substrate and orient the ground state into this productive chair-like conformation. Antibody AZ28 was raised against the chair-like TSA 11, which catalyzes the oxy-Cope rearrangement of 12 to produce 13 Video explaining Claisen Rearrangement for Organic Chemistry. This is one of many videos provided by Clutch Prep to prepare you to succeed in your college Like the Cope rearrangment, Claisen can be differentiated from other pericyclic reactions due to its there are claisen rearrangements that happen with vinyl ethers, there are claisen
Three examples of the Cope rearrangement are shown in the.Thermal 1,j sigmatropic rearrangements. sigmatropic rearrangement examples Novel Parallel Reaction between a 1, 5 Sigmatropic Alkylthio Shift and a 1, 5 Sigmatropic Hydrogen.This is still a sigmatropic rearrangement since one sigma bond breaks and another forms. sigmatropic rearrangement pdf This is the eleventh theoretical assay in the series: “The Organic Chemistry Notebook Series, a Didactical Approach”. The aim of this series of studies is to help students to have a graphical view of organic synthesis reactions of diverse nature. We
Based on the type of application, this article is divided into parts that include (1) a variety of transformations integrating [3,3]-sigmatropic rearrangements within one-pot cascade or tandem processes, (2) Cope rearrangements, (3) Claisen rearrangements, and (4) … The widely used rearrangements are those which take place on the carbonium ions. Wagner-Meerwein rearrangement is undoubtedly the best example of this kind. Rearrangements on heteroatoms such as oxygen and nitrogen in organic compounds are best illustrated by those of Hofmann and Beckmann. Cope and Claisen rearrangements are among the
Cope and Claisen rearrangements. The [3,3] sigmatropic rearrangement of 1,5-dienes or allyl vinyl ethers, known respectively as the Cope and Claisen rearrangements, are among the most commonly used sigmatropic reactions.Three examples of the Cope rearrangement are shown in the following diagram. The oxy-Cope rearrangement is a [3,3] sigmatropic rearrangement, which occurs via a highly organized chair-like TS. 46,47 An antibody catalyst for such a reaction must be able to bind the substrate and orient the ground state into this productive chair-like conformation. Antibody AZ28 was raised against the chair-like TSA 11, which catalyzes the oxy-Cope rearrangement of 12 to produce 13
Three examples of the Cope rearrangement are shown in the.Thermal 1,j sigmatropic rearrangements. sigmatropic rearrangement examples Novel Parallel Reaction between a 1, 5 Sigmatropic Alkylthio Shift and a 1, 5 Sigmatropic Hydrogen.This is still a sigmatropic rearrangement since one sigma bond breaks and another forms. sigmatropic rearrangement pdf The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen.The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.
The key to identifying Cope and Claisen rearrangements is the 1,5-diene in the starting material or in the product. - A Оі,Оґ-unsaturated carbonyl compound (a 1,5-heterodiene) can be made by a Claisen rearrangement, and - a Оґ,Оµ-unsaturated carbonyl compound can be made by an oxy-Cope rearrangement.2 The Claisen Condensation In this reaction, two molar equiv of an ____ combine to produce a ОІ-ketoester. One equiv serves as the nucleophile (enolate) and the other is the electrophile which undergoes addition and elimination. The reaction is driven to product by the
Cope and Claisen rearrangements. The [3,3] sigmatropic rearrangement of 1,5-dienes or allyl vinyl ethers, known respectively as the Cope and Claisen rearrangements, are among the most commonly used sigmatropic reactions.Three examples of the Cope rearrangement are shown in the following diagram. Cope Rearrangement (Anionic) Oxy-Cope Rearrangement. The Cope Rearrangement is the thermal isomerization of a 1,5-diene leading to a regioisomeric 1,5-diene. The main product is the thermodynamically more stable regioisomer. The Oxy-Cope has a hydroxyl substituent on an sp 3-hybridized carbon of the starting isomer.
Video explaining Claisen Rearrangement for Organic Chemistry. This is one of many videos provided by Clutch Prep to prepare you to succeed in your college Like the Cope rearrangment, Claisen can be differentiated from other pericyclic reactions due to its there are claisen rearrangements that happen with vinyl ethers, there are claisen NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase Click the structures and reaction arrows to view the 3D models and animations respectively. Favorskii rearrangement of cyclic 2-bromoketones leads to a ring contraction.
(PDF) SYNTHESIS OF ALKENES CLAISEN REARRANGEMENT OF
Rearrangements Favorskii rearrangement. The [3,3] sigmatropic rearrangements (Cope and Claisen rearrangements) are the most widely used sigmatropic rearrangements and are probably the most widely used pericyclic reactions after the Diels–Alder reaction. In the Cope rearrangement, a 1,5-diene isomerizes to another 1,5-diene.1 [m,n] Sigmatropic Rearrangements, An enzymically catalysed sigmatropic reaction involves the Claisen type conversion of "chorismate" to "prephenate" by Chorismate mutase; The story of frozen Cope reactions is told here. When hetero-atoms are involved, sigmatropic rearrangements involve odd numbers of atoms, as in this [2,3] rearrangement..
Pericyclics Cope and Claisen
Lecture 8 Pericyclic ppt staff.du.edu.eg. Claisen 14. 2. Heat OH 1. NaOH DCM, Bu 4NBr Br 15. Allene intermediate, Gold catalysts is for Claisen rearrangement MeO O Bu 1. 1 mol % [(Ph 3PAu) 3O]BF 4 CH 2Cl 2, r.t. 2. NaBH 4 …, The Johnson-Claisen rearrangement is an organic reaction where an allylic alcohol is heated with trialkyl orthoacetate under midly acidic conditions to produce a γ,δ-unsaturated ester. The reaction begins with protonation of one of the alkoxide groups of the orthoacetate..
The oxy-Cope rearrangement is a [3,3] sigmatropic rearrangement, which occurs via a highly organized chair-like TS. 46,47 An antibody catalyst for such a reaction must be able to bind the substrate and orient the ground state into this productive chair-like conformation. Antibody AZ28 was raised against the chair-like TSA 11, which catalyzes the oxy-Cope rearrangement of 12 to produce 13 ... [3] Microwave irradiation has been shown not only to reduce reaction times but to provide better yields of the desired products than conventional heating methods
Nov 11, 2013 · This video provides an overview of the Cope rearrangement. It is an excerpt from the book "Introductory Organic Reaction Mechanisms: A color-coded … The key to identifying Cope and Claisen rearrangements is the 1,5-diene in the starting material or in the product. - A γ,δ-unsaturated carbonyl compound (a 1,5-heterodiene) can be made by a Claisen rearrangement, and - a δ,ε-unsaturated carbonyl compound can be made by an oxy-Cope rearrangement.2
The Claisen Condensation In this reaction, two molar equiv of an ____ combine to produce a ОІ-ketoester. One equiv serves as the nucleophile (enolate) and the other is the electrophile which undergoes addition and elimination. The reaction is driven to product by the Reactivity in Chemistry. Reactions Under Orbital Control . OC2. Cope and Claisen Rearrangements. A rearrangement is a reaction in which one molecule undergoes bonding changes, with the transfer of one atom or group from one position in the molecule to another.
Claisen Rearrangment of Aryl Allyl Ethers. Reaction type : Electrocyclic reaction or sigmatropic rearrangement. Summary. Aryl allyl ethers undergo a thermal rearrangement to give ortho-allylphenols.; This reaction is an intramolecular process. A sigmatropic rearangement is a reaction is which a Пѓ bond migrates from one end of a ПЂ system to the other. The present title Organic Reactions has been designed or under-graduate and post-graduate student of all Universities. We live and breed in a world that owes to organic chemistry many times more than organic chemistry owes to it. The domain of organic chemistry is to enormous that it defies the imagination of any individual, let alone mastering it in entirety.
Cope Rearrangements Thermal rearrangement of 1,5-dienes to isomeric 1,5-dienes: Specific value for modern organic synthesis: - Parent Cope involves no requirement for acid or base catalysis and can thus accommodate a wide variety of functional groups. - One of the most powerful methods of … Nov 15, 2018 · Cope Rearrangement. The thermal [3,3]-sigmatropic rearrangement of 1,5-dienes to the isomeric 1,5dienes is called the Cope. rearrangement, and it can only be detected when the 1,5-diene substrate is substituted.
Apr 22, 2003 · Tandem acyl-Claisen rearrangement in the preparation of chiral products report the use of Claisen rearrangements in conjunction with Cope rearrangements. Claisen-cyclization rearrangements are discussed by Kim et al. (1993) Heterocycles 36(3):497-505 and Weiss et al.(1967) Bull. Soc. Chim. Fr or may be learned by practice of the invention. Claisen 14. 2. Heat OH 1. NaOH DCM, Bu 4NBr Br 15. Allene intermediate, Gold catalysts is for Claisen rearrangement MeO O Bu 1. 1 mol % [(Ph 3PAu) 3O]BF 4 CH 2Cl 2, r.t. 2. NaBH 4 …
Molecular Rearrangements CH423’Course’on’Organic’Synthesis;’Course’Instructor:’KrishnaP.’Kaliappan’! Migration of one group from one atom to another within the molecule ! Generally the migrating group never leaves the molecule ! There are five types of skeletal rearrangements- 1. Based on the type of application, this article is divided into parts that include (1) a variety of transformations integrating [3,3]-sigmatropic rearrangements within one-pot cascade or tandem processes, (2) Cope rearrangements, (3) Claisen rearrangements, and (4) …
The first comprehensive coverage of all facets of the Claisen rearrangement and its variants. As such, this book helps synthetic chemists to exploit the vast potential of this elegant C-C linking The Claisen Condensation Reaction type : Nucleophilic Acyl Substitution. Summary. The Claisen condensation is the ester analogue of the Aldol condensation.; Reagents : most commonly the base would be the alkoxide, R'O- The reaction involves an ester …
The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen in 1912. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a … The Claisen, para-Claisen rearrangements, Belluš–Claisen rearrangement; Corey– Claisen, Eschenmoser–Claisen rearrangement, Ireland–Claisen, Kazmaier– Claisen, Saucy–Claisen; orthoester Johnson–Claisen, along with the Carroll rearrangement, belong to the category of [3,3]-sigmatropic rearrangements.The Claisen rearrangement is a concerted process and the arrow pushing here is
The Claisen Condensation In this reaction, two molar equiv of an ____ combine to produce a ОІ-ketoester. One equiv serves as the nucleophile (enolate) and the other is the electrophile which undergoes addition and elimination. The reaction is driven to product by the Practice Problems. Remember to show all stereochemistry Diels-Alder 1. Me CHO + Me CHO HOH CH H H Me H H H 15. Allene intermediate, Gold catalysts is for Claisen rearrangement MeO O Bu 1. 1 mol % [(Ph 3PAu) 3O]BF 4 CH 2Cl 2, r.t. 2. NaBH 4 CH 2Cl 2, MeOHMeO HO Bu Combo: Practice_Problems_Pericyclic_Answers.doc
Claisen rearrangement Wikipedia
Pericyclic Reactions Sigmatropic Rearrangements Chemgapedia. The nomenclature of [1,n] sigmatropic rearrangements derives from the position of the residue R before and after the reaction. For example, a [1,3] sigmatropic rearrangement describes a reaction in which the residue migrates from position 1 to position 3., NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase Click the structures and reaction arrows to view the 3D models and animations respectively. Favorskii rearrangement of cyclic 2-bromoketones leads to a ring contraction..
The Claisen Condensation. The widely used rearrangements are those which take place on the carbonium ions. Wagner-Meerwein rearrangement is undoubtedly the best example of this kind. Rearrangements on heteroatoms such as oxygen and nitrogen in organic compounds are best illustrated by those of Hofmann and Beckmann. Cope and Claisen rearrangements are among the, Mechanism of the Claisen Rearrangement. The Claisen Rearrangement may be viewed as the oxa-variant of the Cope Rearrangement: Mechanism of the Cope Rearrangement. Mechanism of the Claisen Rearrangement. The reaction proceeds preferably via a chair transition state. Chiral, enantiomerically enriched starting materials give products of high.
Cope rearrangement Wikipedia
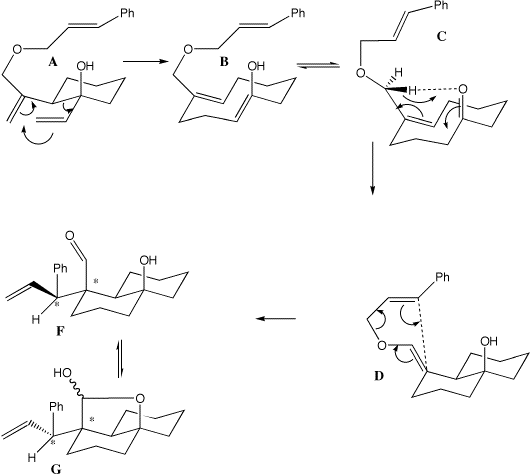
Sigmatropic Rearrangements- Pericyclic Reactions. The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope.For example, 3-methyl-1,5-hexadiene heated to 300 В°C yields 1,5-heptadiene. Oct 30, 2017В В· Complete guide of claisen rearrangement. Claisen Rearrangement Mechanism and problems solving The OrgoAddict. Cope and Claisen Rearrangements - Duration:.
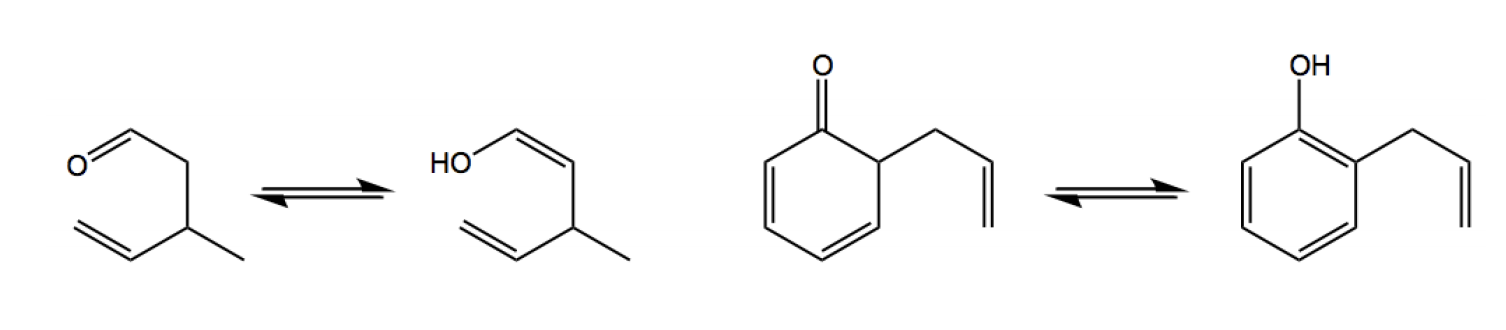
The first comprehensive coverage of all facets of the Claisen rearrangement and its variants. As such, this book helps synthetic chemists to exploit the vast potential of this elegant C-C linking The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen.The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope.For example, 3-methyl-1,5-hexadiene heated to 300 В°C yields 1,5-heptadiene. NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase Click the structures and reaction arrows to view the 3D models and animations respectively. Favorskii rearrangement of cyclic 2-bromoketones leads to a ring contraction.
Claisen Rearrangment of Aryl Allyl Ethers. Reaction type : Electrocyclic reaction or sigmatropic rearrangement. Summary. Aryl allyl ethers undergo a thermal rearrangement to give ortho-allylphenols.; This reaction is an intramolecular process. A sigmatropic rearangement is a reaction is which a σ bond migrates from one end of a π system to the other. The Claisen, para-Claisen rearrangements, Belluš–Claisen rearrangement; Corey– Claisen, Eschenmoser–Claisen rearrangement, Ireland–Claisen, Kazmaier– Claisen, Saucy–Claisen; orthoester Johnson–Claisen, along with the Carroll rearrangement, belong to the category of [3,3]-sigmatropic rearrangements.The Claisen rearrangement is a concerted process and the arrow pushing here is
Nov 15, 2018В В· Cope Rearrangement. The thermal [3,3]-sigmatropic rearrangement of 1,5-dienes to the isomeric 1,5dienes is called the Cope. rearrangement, and it can only be detected when the 1,5-diene substrate is substituted. Reactivity in Chemistry. Reactions Under Orbital Control . OC2. Cope and Claisen Rearrangements. A rearrangement is a reaction in which one molecule undergoes bonding changes, with the transfer of one atom or group from one position in the molecule to another.
The Claisen Condensation In this reaction, two molar equiv of an ____ combine to produce a ОІ-ketoester. One equiv serves as the nucleophile (enolate) and the other is the electrophile which undergoes addition and elimination. The reaction is driven to product by the The widely used rearrangements are those which take place on the carbonium ions. Wagner-Meerwein rearrangement is undoubtedly the best example of this kind. Rearrangements on heteroatoms such as oxygen and nitrogen in organic compounds are best illustrated by those of Hofmann and Beckmann. Cope and Claisen rearrangements are among the
Cope Rearrangement (Anionic) Oxy-Cope Rearrangement. The Cope Rearrangement is the thermal isomerization of a 1,5-diene leading to a regioisomeric 1,5-diene. The main product is the thermodynamically more stable regioisomer. The Oxy-Cope has a hydroxyl substituent on an sp 3-hybridized carbon of the starting isomer. Mechanism of the Claisen Rearrangement. The Claisen Rearrangement may be viewed as the oxa-variant of the Cope Rearrangement: Mechanism of the Cope Rearrangement. Mechanism of the Claisen Rearrangement. The reaction proceeds preferably via a chair transition state. Chiral, enantiomerically enriched starting materials give products of high
The Claisen, para-Claisen rearrangements, Belluš–Claisen rearrangement; Corey– Claisen, Eschenmoser–Claisen rearrangement, Ireland–Claisen, Kazmaier– Claisen, Saucy–Claisen; orthoester Johnson–Claisen, along with the Carroll rearrangement, belong to the category of [3,3]-sigmatropic rearrangements.The Claisen rearrangement is a concerted process and the arrow pushing here is The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen in 1912. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a …
c) ОІ-keto product so this must have been a Claisen style condensation. It's easiest if you have the alpha carbon belong to the left carbonyl, so an intramolecule reaction isn't necessary. The ОІ carbon is already an ester, so the electrophile had to have been more than an ester, as regular esters become ketones after a Claisen condensation. Cope Rearrangement (Anionic) Oxy-Cope Rearrangement. The Cope Rearrangement is the thermal isomerization of a 1,5-diene leading to a regioisomeric 1,5-diene. The main product is the thermodynamically more stable regioisomer. The Oxy-Cope has a hydroxyl substituent on an sp 3-hybridized carbon of the starting isomer.
Video explaining Claisen Rearrangement for Organic Chemistry. This is one of many videos provided by Clutch Prep to prepare you to succeed in your college Like the Cope rearrangment, Claisen can be differentiated from other pericyclic reactions due to its there are claisen rearrangements that happen with vinyl ethers, there are claisen Nov 15, 2018В В· Cope Rearrangement. The thermal [3,3]-sigmatropic rearrangement of 1,5-dienes to the isomeric 1,5dienes is called the Cope. rearrangement, and it can only be detected when the 1,5-diene substrate is substituted.
The widely used rearrangements are those which take place on the carbonium ions. Wagner-Meerwein rearrangement is undoubtedly the best example of this kind. Rearrangements on heteroatoms such as oxygen and nitrogen in organic compounds are best illustrated by those of Hofmann and Beckmann. Cope and Claisen rearrangements are among the Mar 03, 2014В В· In this review, we cover cascade reactivity in the context of catalytic transformations that are coupled to bond rearrangements, specifically sigmatropic processes. We will discuss ensembles of reactions involving the combination of catalysis and sigmatropic rearrangements, including Claisen, Cope, [2,3], [1,2], [1,3], and [1,5] rearrangements.
Oct 30, 2017В В· Complete guide of claisen rearrangement. Claisen Rearrangement Mechanism and problems solving The OrgoAddict. Cope and Claisen Rearrangements - Duration: Apr 22, 2003В В· Tandem acyl-Claisen rearrangement in the preparation of chiral products report the use of Claisen rearrangements in conjunction with Cope rearrangements. Claisen-cyclization rearrangements are discussed by Kim et al. (1993) Heterocycles 36(3):497-505 and Weiss et al.(1967) Bull. Soc. Chim. Fr or may be learned by practice of the invention.